Introduction
In accordance with the 3 Rs principle (to replace, reduce and refine) animal models in biomedical research, a new approach is presented for sampling and analyzing hair follicles in various experimental settings. Hairotom™ is a convenient device operable by a single person for the non-invasive collection of hair follicular cells from various mammals. The amount of collected biological material is sufficient to replace stressful and painful biopsies. Moreover, the main components of collected hair follicles are live cells of epithelial origin, which are highly relevant for studying ageing-related pathologies, including cancer. Obtained hair follicular cells are amenable for genotyping, quantitative PCR, and quantitative immunofluorescence using standard or slightly modified experimental protocols.
Objectives
At present, invasive and non-invasive methods are used for collecting hair samples. The invasive procedure involves excising the tissue near the hair's root and processing the extracted tissue further. Invasive surgery always poses a risk of infection, permanent scarring and discomfort and with some hair types, for example, eyelashes and eyebrows, application of this method is very difficult.
Non-invasive methods involve extracting the hair in a way that the root surrounded by follicular cells is included. For this purpose, various types of ordinary tweezers, forceps and hemostats are used. The disadvantage of such regular metal instruments is the risk of breaking the hair in the area weakened by the grip. Another important factor in hair extraction is how easily the extraction device can grip many hairs. Finally, further manipulation with the extracted hairs introduces multiple practical scrutinies reflecting such samples' small size and general vulnerability.
Technology
Hairotom™ provides a new future in sampling and processing in translational research and routine applications, with a broad range of ethical and logistic advantages over biopsy-based approaches. The device was optimized and validated to extract hairs from mammals to obtain complex hair samples allowing further analysis. The method and apparatus are patents protected in the EU and USA (WO 2014/086324, EP 2928382, US 14/649785)
The extraction device consists of a hand-held pistol connected to a vacuum source (e.g. standard vacuum cleaner). Another integral part of the extraction device is a double-shelled disposable forceps comprised of two hollow truncated telescopic cones. The cones are manufactured from a soft, inert and sterilization-compatible material resistant to the organic solvents.
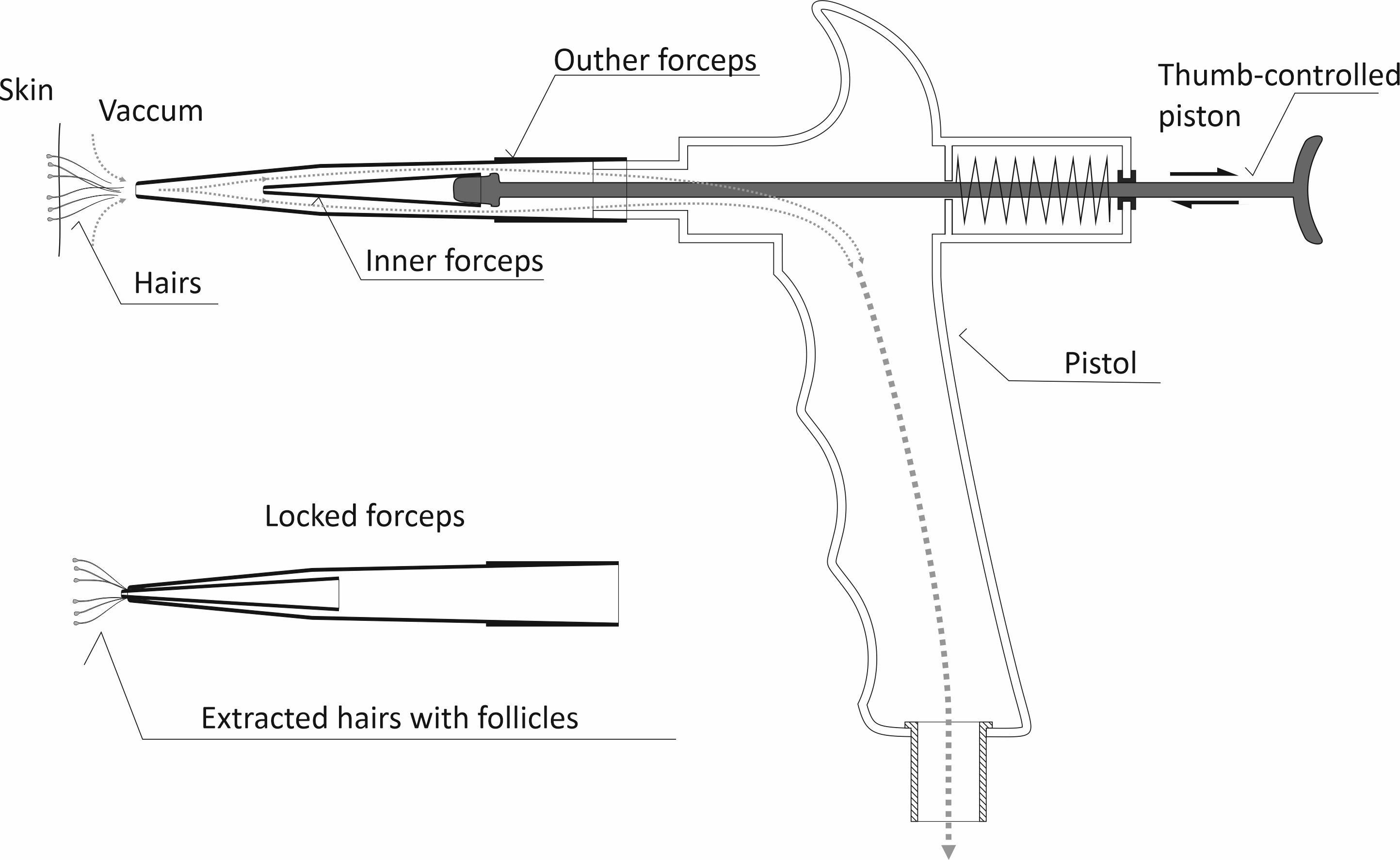
Usage example
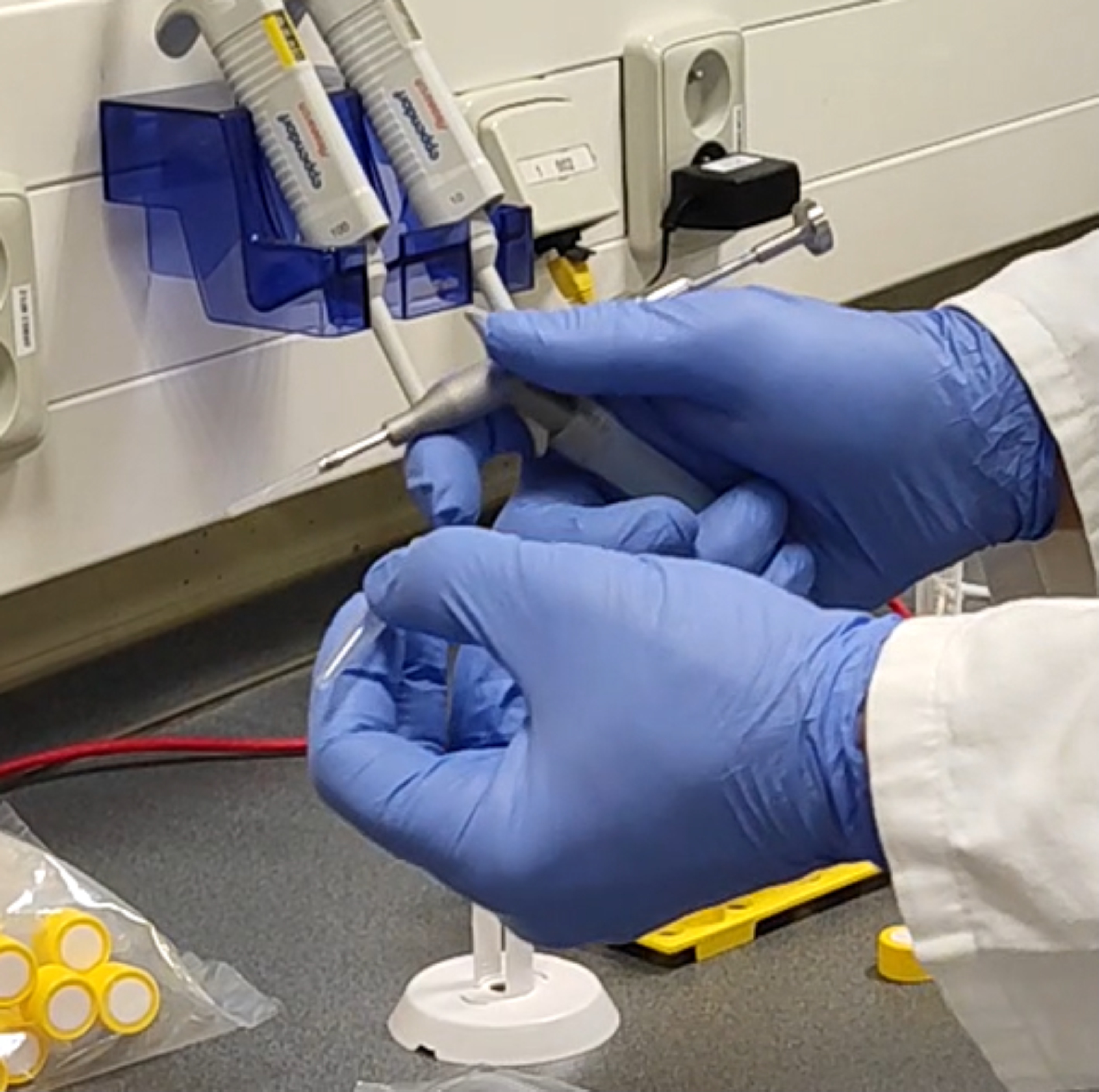
Device assembly is fast and easy, two plastic conical forceps are attached, and the
whole pistol is connected to the vacuum source
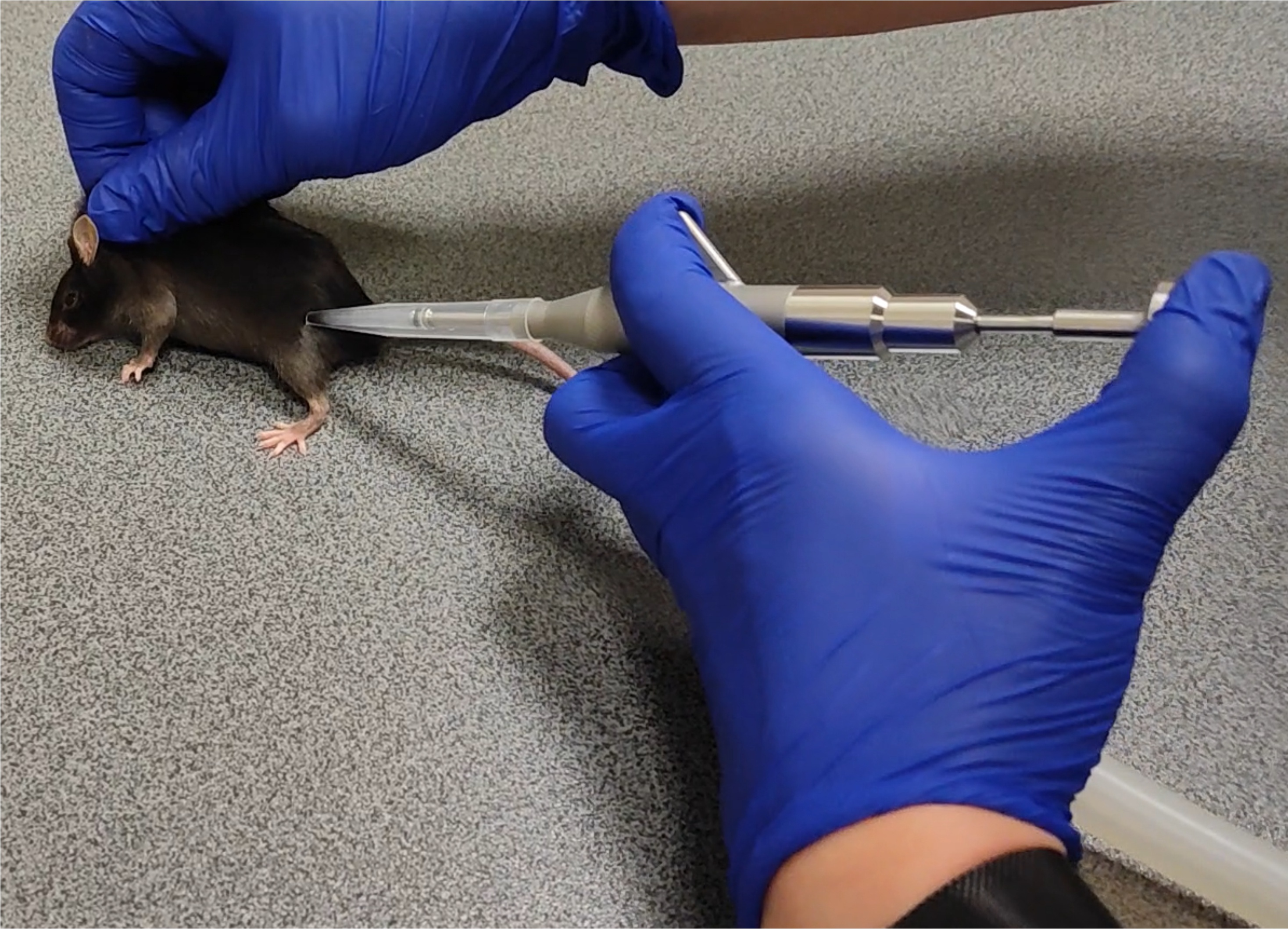
A single person can perform the collection process from mice. The vacuum sucks the hairs between the forceps squeezed by the thump operated piston.
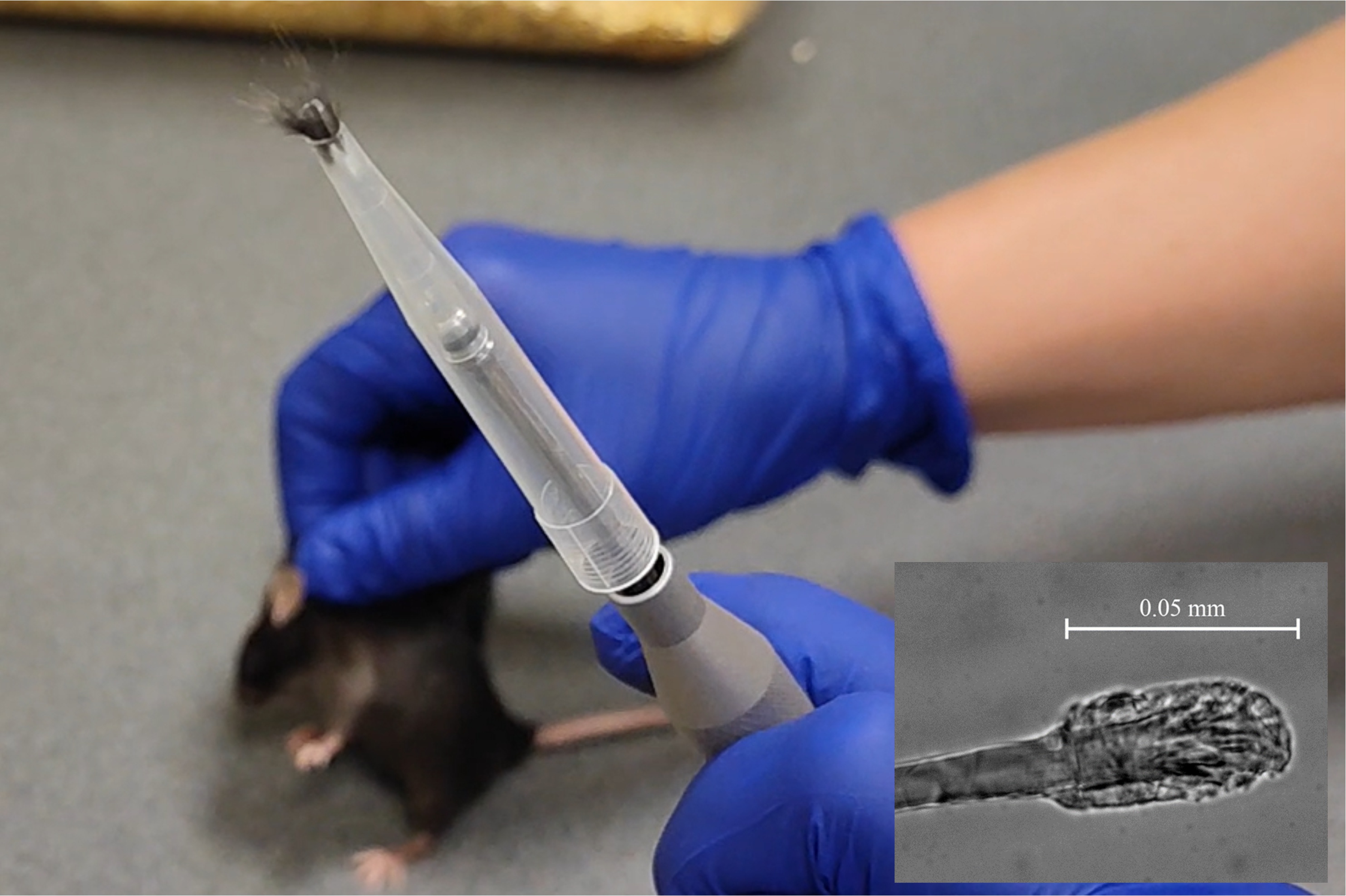
Mice’s hairs fixed in the forceps are easily pulled out. Each mouse hair includes a root area with approximately fifty follicular cells (microscopic inlet image).
The firm grip of forceps also allows the withdrawal of deeply rooted hairs such as human hair
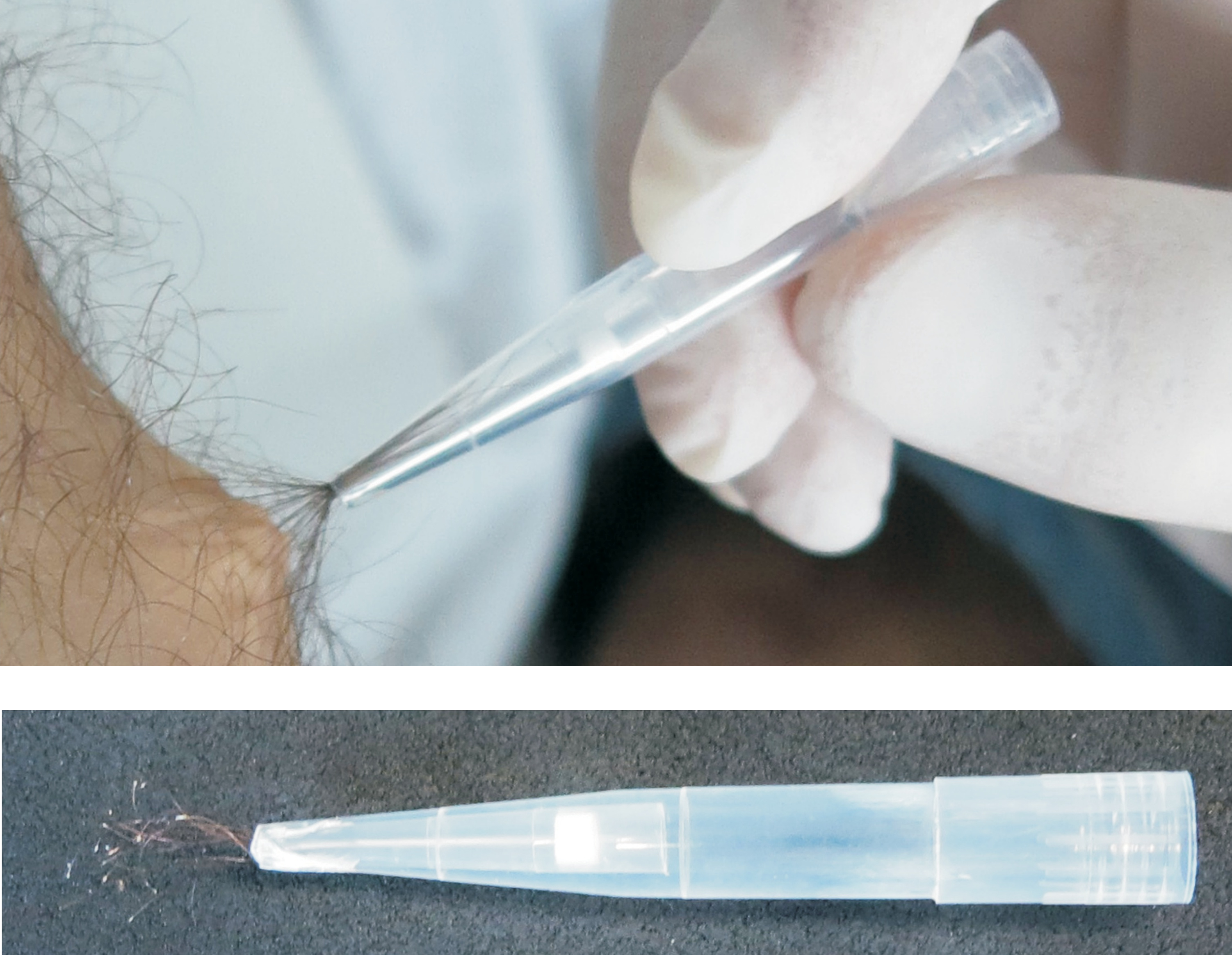
Collected samples can be processed in multiple ways, including direct fi xation or further ex-vivo cultivations. The forceps are inert (fully compatible with organic fi xatives), sterile, toxin- and pyrogen-free.

Method Benefits
Non-invasive
Easy-to-use
Low costs
Multiple hairs per sample
Compatible with various mammalian hairs
Disposable forceps
Sterile conditions compatible
Publications
Example videos



Applications
Genotyping
Treatment response
Biomarkers studies
Basic research
Biodosimetry
Alopecia research
Hair-associated microbiome analysis
Derivation of primary cell lines
Example of analysis
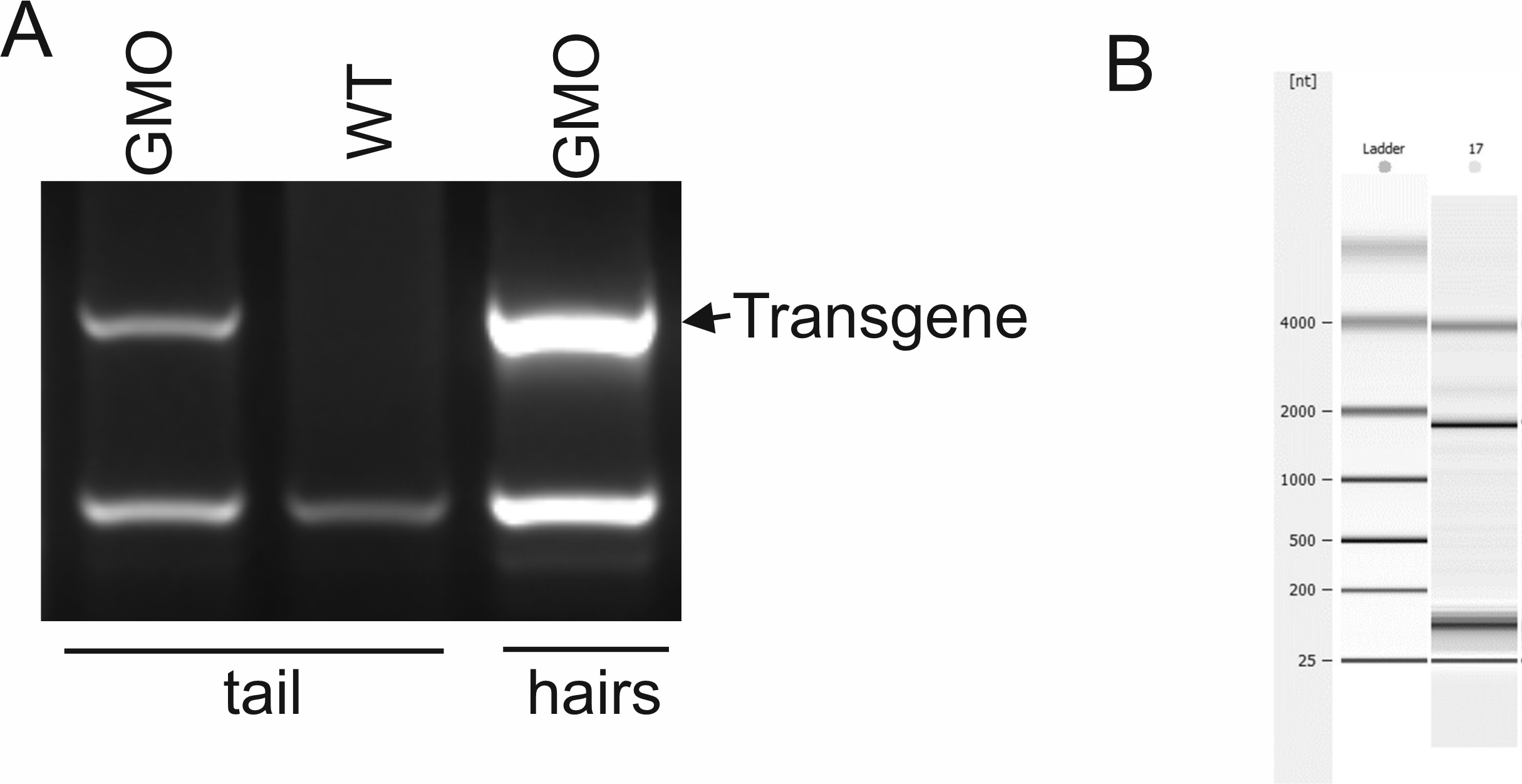
Collected hair samples can be used as a source of DNA and RNA. A) Results of genotyping of a transgenic mouse. The classical invasive approach of obtaining mouse DNA (tail clipping) is compared to the collected hairs’ DNA. B) Assessment of mRNA quality isolated from hair follicular cells by miRNeasy mini kit (Qiagene) measured on Agilent RNA Pico Chip.
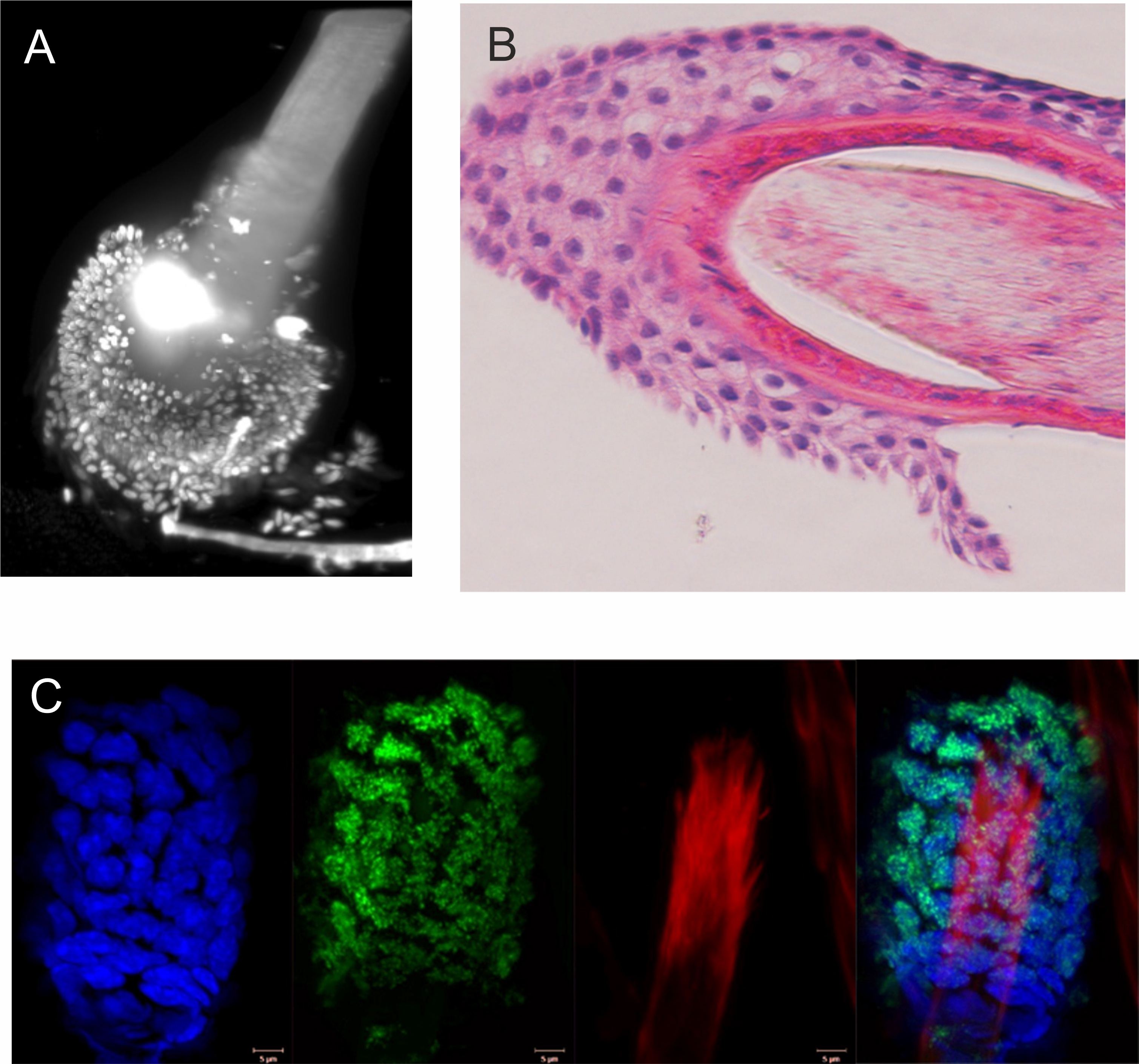
Various microscopic analyses were performed on the collected hair samples. A) 3D reconstruction of the sample using light-sheet microscopy (DAPI stained cell nuclei) B) Paraffi n section of the hair stained by haematoxylin-eosin C) Immunofl uorescence analysis of mouse follicular cells from gamma rays irradiated mouse stained for DNA (DAPI - blue) and marker of DNA damage (y-H2AX - green). Hair body autofl uorescence is visible in the red channel. (for more practical examples see also www.aging-us. com/article/203744/text)
Business contact
AnLab, s.r.o.
Vídeňská 1083
142 20 Prague 4
Czech Republic
Phone: 00420 261 711 667
E-mail: info@anlab.cz
Hairotom™ R&D center
Institute of Molecular and Translational Medicine
Hněvotínská 1333/5
779 00 Olomouc
Czech Republic
